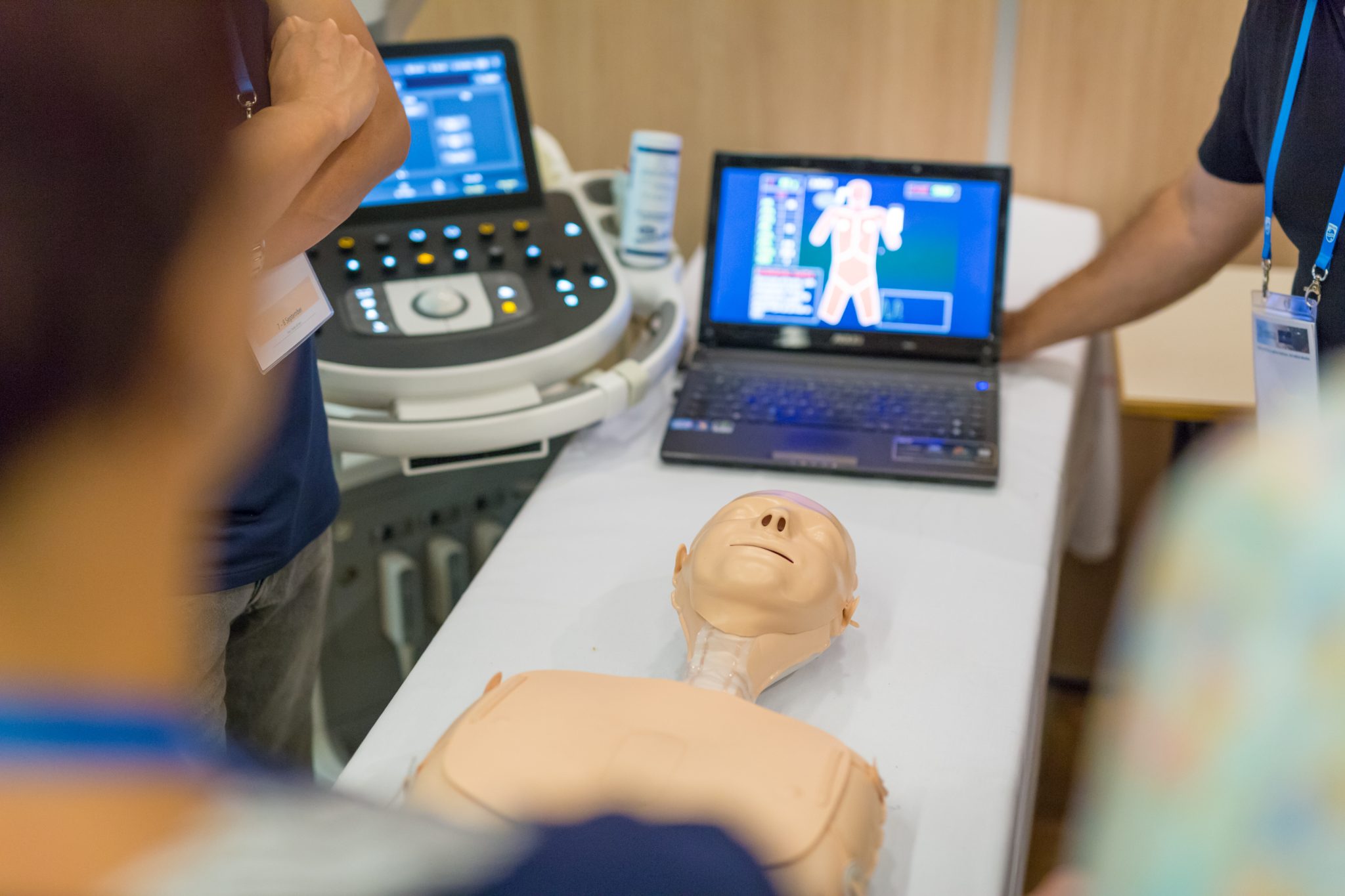
Experience Sago’s Legacy of Impact
Our expertise in participant recruiting and clinical project execution is backed by our rigorous clinical Standard Operating Procedures and over 50 years as an industry-leading research provider. Sago provides unparalleled access to your target audiences for data you can trust.
Clinical Recruitment Capabilities
Diverse online and offline recruiting methods enable a vast respondent pool from which to collect your data. Our vast proprietary panels of opted-in consumers and patients support more than 250,000 participants in our clinical studies annually.
Broad Therapeutic Capabilities
Work with trusted professionals in healthcare research across all therapeutic areas, with expertise in cardiovascular conditions, dermatology, endocrinology, gastroenterology, genetic disease, immunology, neurology, oncology, and rheumatology.
![]()
One Dedicated Point of Contact
At Sago, you’ll have a dedicated Clinical Project leader to oversee the recruitment and completion of your clinical research protocols. Certified teams and proprietary sites provide study consistency and quality to your timeline.

Integrated Data Collection Sites
Experience world-class Sago insights, testing, and simulation sites across the US and Europe. We coordinate all aspects of clinical research in each location with consistently high quality.

Regulated Data Collection Expertise
Our clients trust our protocols for FDA pre-market product approvals. Our expert team works with you to collect and deliver reliable, actionable data.
What Sets Sago Apart?

Patient Panel
- Over 400,000 active members
- Tracks over 326 medical conditions

Consumer Panel
- Over 700,000 active members
- Deeply profiled verified members

High-level recruitment capabilities
Shorten your research timeline
Types of Clinical Research We Undertake
- Low Literacy/ Low Health Literacy
- Invasive & Non-Invasive Devices
- Claims Substantiation
- Actual Use Tests (AUT)
- Label Comprehension
- Tracking Studies
- Human Factors Testing
- Product Testing
- Observational Studies
- User Experience
- HEOR/RWE
- Cognitive Testing
- Packaging Design
- Rx-to-OTC Switch
- Cognition Testing
- OTC Products
An Expert Clinical Team
- Dedicated Clinical Project Management Team
- Approved Government Vendor
- Trained in Human Subjects Protection and Good Clinical Practice
- Principal Investigators experienced with protocol design and IRB submissions
- Proven standards of discipline and attention to detail in our management and audit processes
- Customized solutions for each study to optimize success
- Consultative partnership
- Consistent, reliable, and transparent in our unified SOPs
- Scalable to your project scope
Teams We Support
- CROs
- Manufacturers – CPG, Device, Drug
- Research & Development
- Product Design & Engineering
- Insights Agencies
- Human Factors Groups/Agencies
- Health Economics and Outcomes Research (HEOR)
- Real World Evidence Researchers
- Universities & Academic Institutions
- Government Agencies
We Keep Good Company
Our memberships and acredidations.